An editorial published in the JAMA the 11 of February, summarize all data currently available on the epidemic situation of the Ebola virus in West Africa and on treatment available or in clinical trial.
Current epidemiology:
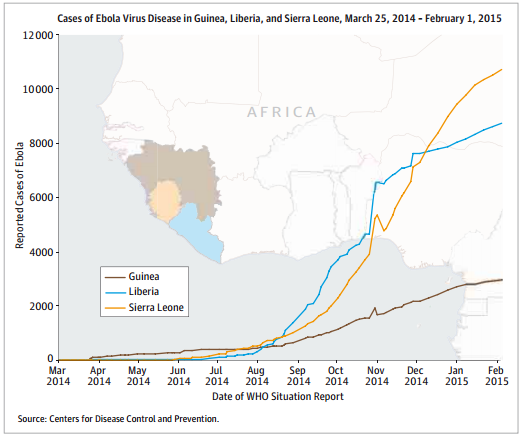
In late January, the WHO announced that the Ebola epidemic had slowed. Inger Damon MD, PhD at the CDC said “Things are currently trending in a good direction, suggesting growing control”. But, vigilance is necessary
to prevent resurgence and Brice de la Vingne from MSF said justly that a single new case is enough to reignite
an outbreak.
Treatment progresses:
Currently, Currently, the most effective treatment for Ebola virus disease is fluid and electrolyte replacement and
other supportive care.
ZMAPP: 3 different neutralizing antibodies against the Ebola virus, based on promising results from animal studies. It has now are being fast tracked into phase 1 and 2 clinical trials in US and Liberia. Pharmaceutical companies are involved in the project to help scale up antibody production.
TKB-Ebola: blocks the enzyme that catalyzes the replication of the Ebola virus, is currently in phase 1
Favipiravir et brincidofovir: have been launched at the end of 2014 by MSF
Blood and plasma from patients who survived an Ebola virus infection: Multiple clinical trials are currently underway in the affected countries. A recent analysis projected that transfusion therapy could save 151 to 3586 lives but with a risk of transmission of other diseases.
Vaccines:
Vaccin developed by NIAID and GlaxoSmithKline: uses chimpanzee adenovirus type 3 (ChAd3) as a vector
to deliver noninfectious Ebolagenes into the body to stimulate an immune response. Preliminary results from a phase 1 trial of the ChAd3 vaccine in 60 health volunteers in United Kingdom reported no safety concerns and found that the vaccine stimulated an immune response not as robust as expected. But the authors suggest this
might be remedied by adjusting the vaccine dose.
The US Department of Defense, NewLink and Merck: phase 1 trials of another vaccine that uses vesicular stomatitis virus (VSV) as a vector. A phase 2-3 randomized double-blind trial that will compare both the VSV and ChAd3 Ebola virus vaccines and a placebo in 27 000 health workers and others at risk in Liberia
The CDC is also preparing for a 6000-person vaccine trial in Sierra Leone.
Current difficulties:
Public health organisation of the 3 counties are totally destabilized, populations leaving in the forest are difficult to make them aware, and difference in term of religions and cultures…
Another major factor is the genetic changes in the Ebola virus which can influence clinical trial results.